OUR MISSION
Continuous healthcare innovation that delivers high-impact outcomes at most affordable costs.
01
Impact at Scale
Cardiac Design Labs (CDL) was founded with a vision to create world-class medical devices and platforms for advanced monitoring, diagnostics and compassionate care to the masses.
02
Culture of Research and Development
At CDL, the team is driven by a shared passion for making a difference in the lives of as many patients as possible. We believe the application of cutting-edge technology and the latest advances in medical science can drive more accurate, proactive, and compassionate care for patients anywhere in the globe.
03
Quality obsessed
Over the last decade, we have designed and built patient and cardiac care products of clinical-grade accuracy and globally competing standards in diagnostics and monitoring, while also ensuring highest levels of comfort for the patient and best use of care taker’s time.
OUR VALUES
At Cardiac Design Labs, our values are at the heart of everything we do.
A PEEK INTO OUR JOURNEY
Our history of process and innovation
Inception
CDL was founded in a garage in Whitefield, Bangalore (India) by Sashi Kumar and Anand Madanagopal, with a mission to develop cardiology specific solutions, for early detection of cardiac conditions and thereby save lives through timely detection and treatment of cardiac related abnormalities.
First Prototype - Trial at KMC Manipal
With a grant from the Department of Scientific and Industrial Research (DSIR, Govt of India) CDL developed the first prototype cardiac monitors. These monitors comprised of a body strapped device and a bedside display monitor, that displayed in real-time the ECG, and also undertook basic data analysis from such readings.
The first version of algorithms was launched, to handle ambulatory ECG for baseline correction, beats, ECG segments and all basic rhythms.
Winner Grand Jury Prize at Startup India
CDL was one of the five startups selected to participate in the Google launchpad of the first Startup India Event, where The Honourable Prime Minister, launched the India Startup Policy. CDL was awarded the Grand Jury Prize due to our technology innovation and its potential to impact lives at scale.
Holter Services at Hospitals
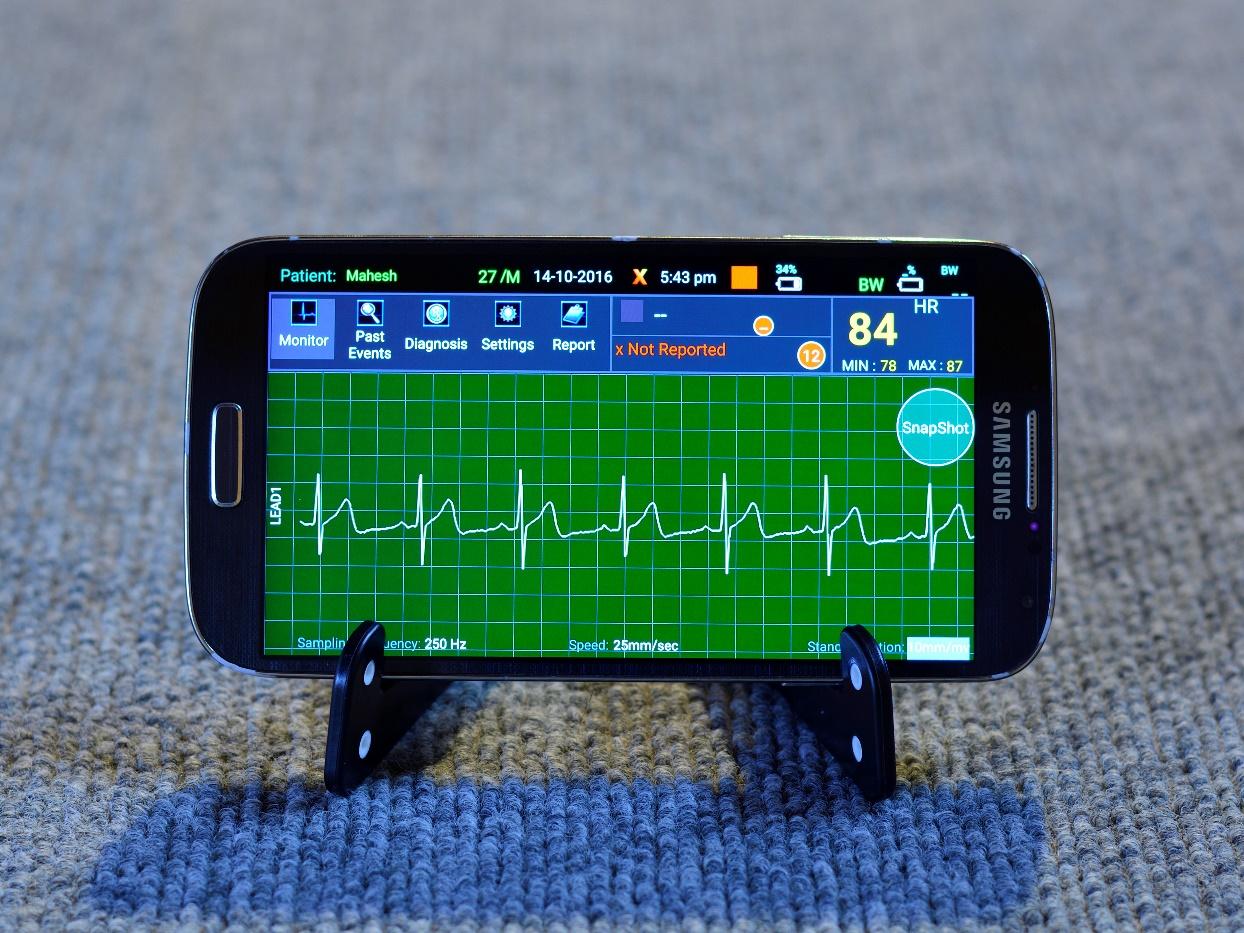
With the body worn device and its ability to record and analyse continuous ECG for 24 hours, we launched Holter Services in hospitals. The device was a wireless device transmitting ECG in real-time to a phone, wherein it was signal corrected and analysed for anomalies.
Launch of Holter Analysis and Viewing Systems
In the context of Holter services, while a connected Holter detected events, the need to undertake processing as in a conventional Holter on a Personal Computer (PC) with comprehensive tracing needed to be addressed. In record time CDL developed and launched developed the based software for comprehensive analysis.
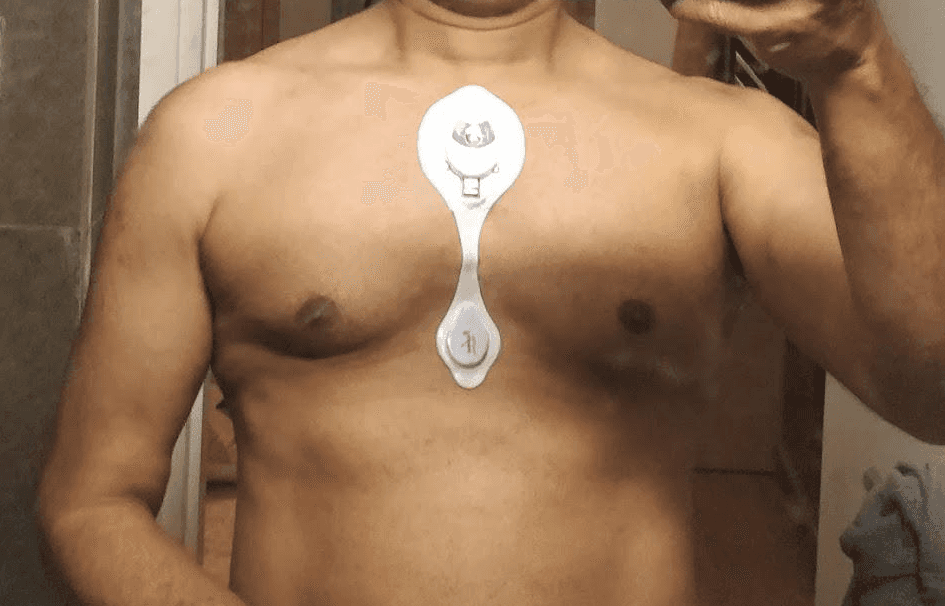
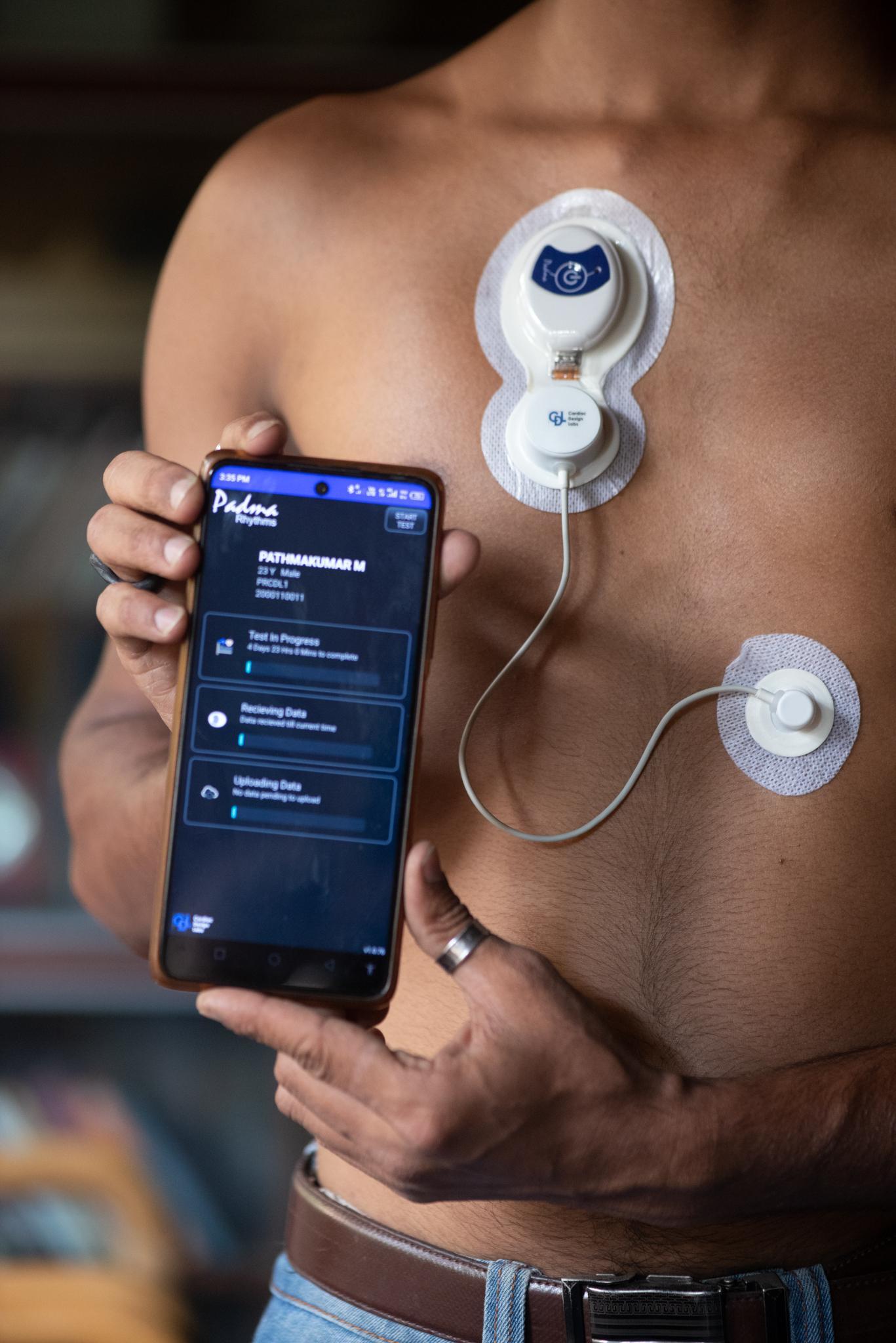
First Prototype of Padma Rhythms®
As the world was moving towards smaller wearables, we evolved our version for a smaller device, with a patch. This prototype was undertaken with the aim to maintain the Holter’s high quality ECG outputs through leveraging the benefits of a patch.
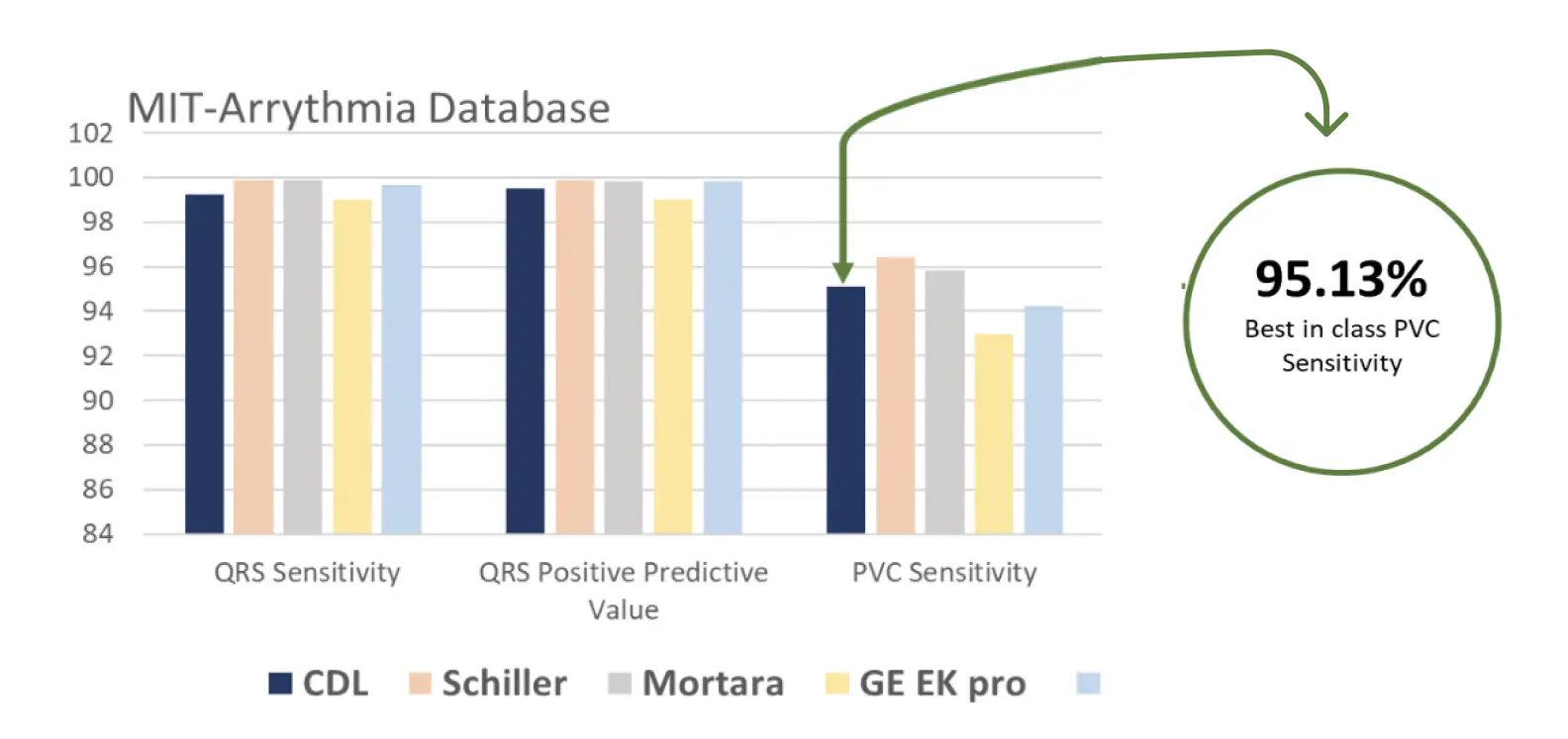
Validated Algorithms - Benchmark against global standards
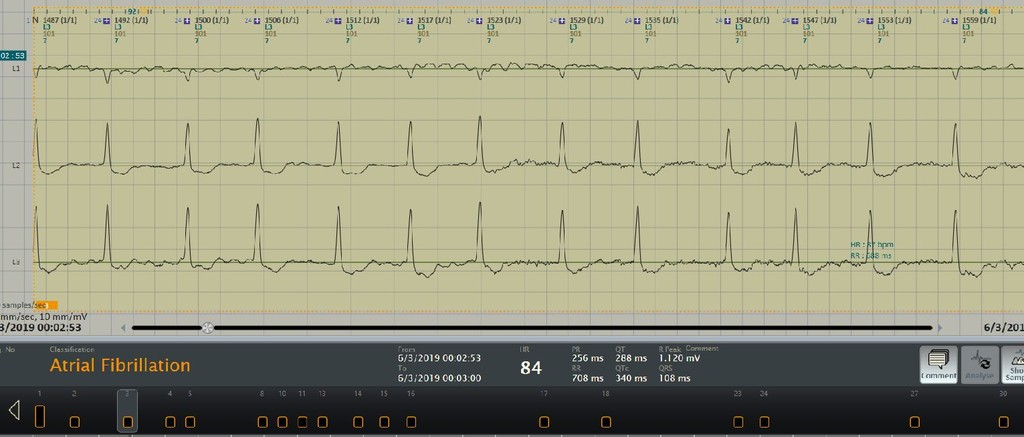
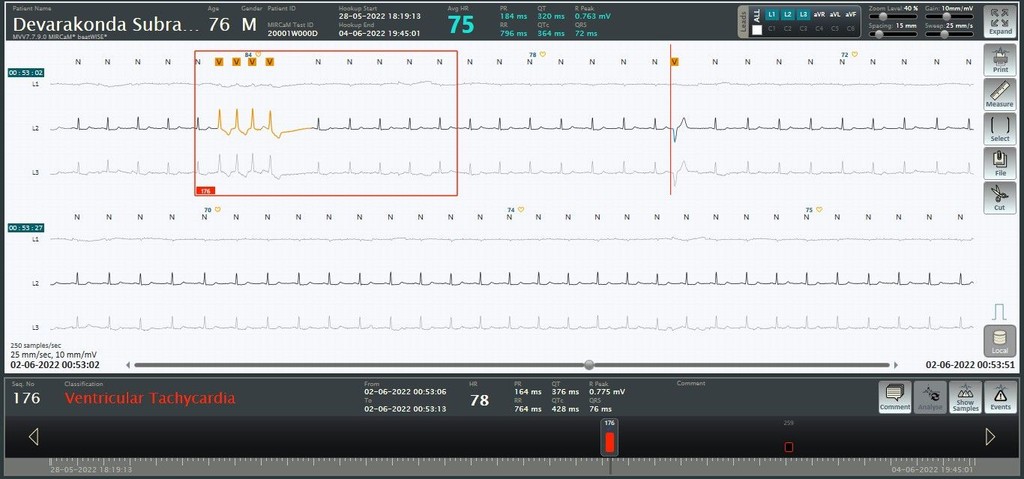
A significant component of the system is the algorithm results benchmarked on the reference databases against some of the global players. The system is validated against the MIT arrhythmia database and field streams and we have an impressive accuracy of 95.13 % for PVC Sensitivity which is better than that of global players.
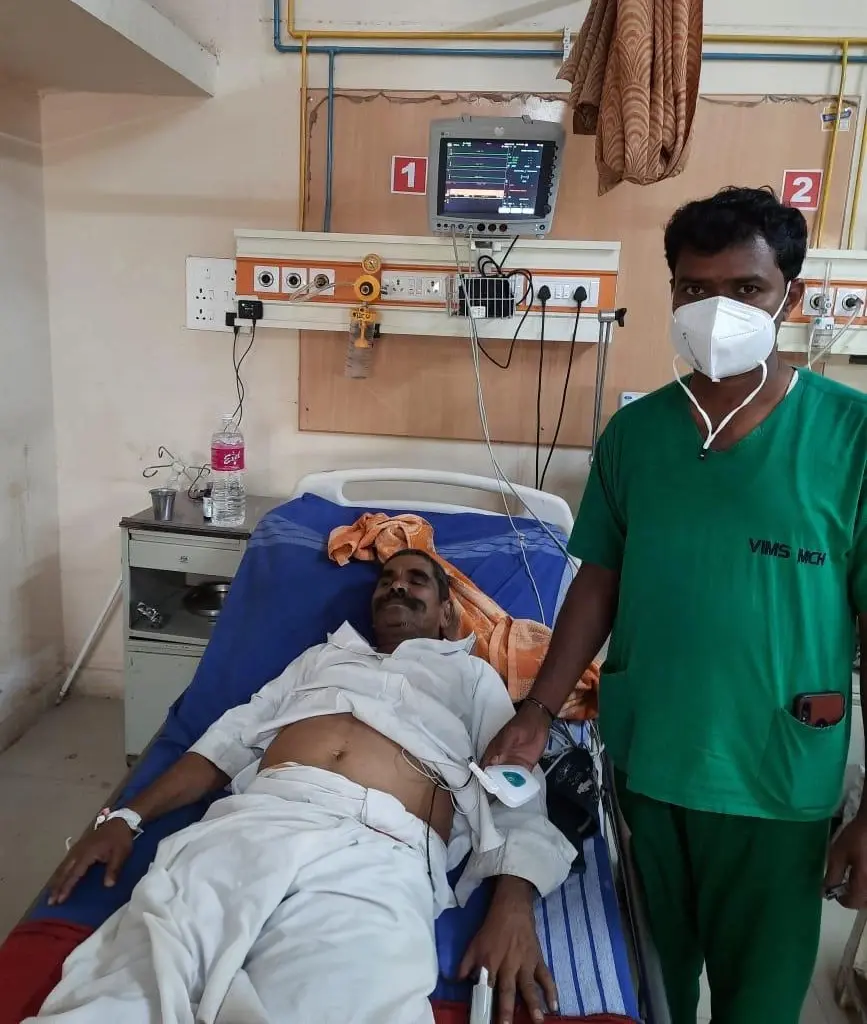
First trial of Padma Vitals®
Padma Vitals was conceived just prior to COVID. Our MIRCaM® platform was extended to accommodate additional sensor data to provide monitoring of all vital parameters. Our first hospital trial was with a palm sized wireless device with central monitoring.
Launch of Padma Vitals® at Hospitals for COVID
CDL was amongst the first companies to launch centralised remote monitoring of vitals for wards that are isolated to treat COVID patients. This ensured limited physical contact of healthcare workers with covid patients. The system alerts nurses and prioritizes their attention and focus towards patient that need immediate nursing care. Such monitoring installed in 5 hospitals during covid. The largest was in NH, monitoring over 100 patients simultaneously.
Padma Vitals® at 2 ICUs in Jayadeva Hospitals
We installed Padma Vitals® in 2 ICUs of SJICR (Jayadeva Hospital) for central and remote monitoring. These were installed as part of the ACT grants.
First trial of Padma Rhythms®
After a year of design iterations, a trial was undertaken on 50 patients to evolve the product towards a production version. Research included design elements to handle high sweating conditions, while being waterproof to handle bathing. The patch had to pass a test to last for 5-7 days on the skin, while transferring data in near real-time without user intervention.
Inauguration of manufacturing rooms
Cardiac Design Labs celebrated the grand inauguration of its state-of-the-art manufacturing rooms, pioneering the future of medical device production with cutting-edge technology and innovation.

OUR TEAM
Meet the people who make it happen
We are a bunch of passionate folks, developing innovative medical devices and solutions that transform the way healthcare providers diagnose and treat heart disease.
Trusted by 300+ Experts